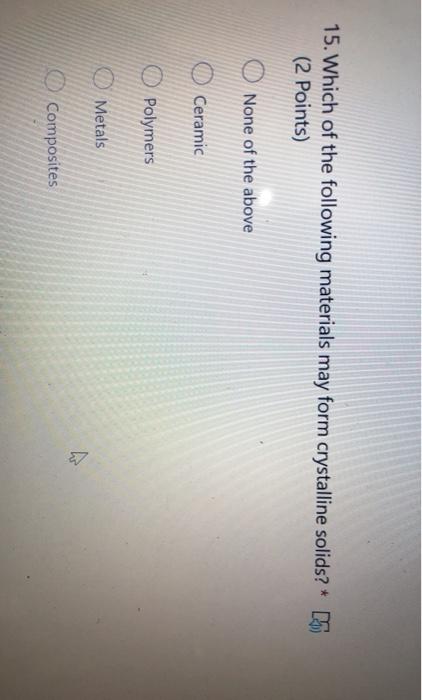
Which of the Following Materials May Form Crystalline Solids
So they are non-crystalline. Some of the examples of molecular crystals are.

Crystalline And Amorphous Solids Explanation Differences Examples Etc
In addition macroscopic single crystals are usually identifiable by their geometrical shape consisting of flat faces with specific characteristic orientations.
. Which of the materials may form crystalline solids. The most common example of an amorphous solid is Glass. It occurs naturally in this form and is the most stable form of.
No polarity is found in the bonds amongst these solids as the same atoms or molecules are joined like Cl 2 one chlorine atom is bonded to another by. Which of the following materials may form crystalline solids. Which of the following materials may form crystalline solids.
Metals polymers and ceramics when subjected to solidification condition for crystallization they all have the ability to form crystalline solids. These solids have weak Van Der Waals forces so they are soft. Which of the following bonding belongs to ceramic materials may be more than one answer.
Crystalline ceramics are harder and more brittle than metals because ceramics have fewer slip systems and. Aionic b metallic c hydrogen d covalent. Gels plastics various polymers wax thin films are also good examples of amorphous solids.
None of the above Under normal solidification conditions all three of these material types may form crystalline solids. Which of the following is the best example of material processing. Such solids consist of atoms arranged in a particular fashion.
Crystalline Glassy Annealing Elastic Modulus Thermal Expansion Coefficient. Metals Ceramics Polymers All the these None of the these. Hence the correct option will be D all of the above.
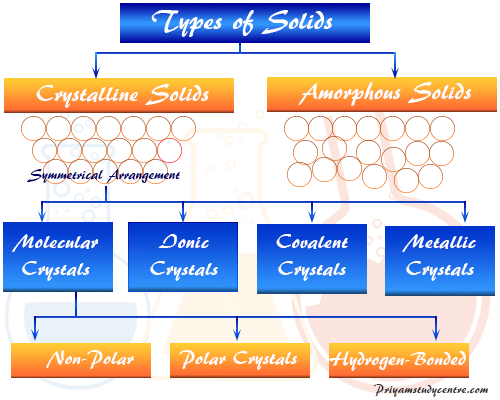
All these types of crystalline solids exhibit different physical properties and are formed by different types of intermolecular forces of attraction. See the answer See the answer done loading. Non-metallic solids like sulphur phosphorus iodine are Crystalline Solids.
All the these Polymers None. The transition to liquid called melting is sharp and transparent as crystalline solids are heated. Substances that consist of large molecules or a mixture of molecules whose movements are more restricted often form amorphous solids.
Metals and ionic compounds typically form ordered crystalline solids. They do not have a geometric shape. In fact just because molecular solids form due to a number of different molecules as a result these types of crystalline solids have variable hardness variable brittleness as well as variable melting points.
All of the above E. Which of the following materials may form crystalline solids. Amorphous solids are rigid structures but they lack a well-defined shape.
Sulphur phosphorus carbon and tin are some important examples of elements which show allotropy. Which of the following materials may form crystalline solids. Crystalline solids are made of stone wood paper and cloth.
Hydrogen H 2 Water H 2 O Ammonia NH 3 Iodine I 2 etc. Graphite is a crystalline form of the element carbon with its atoms arranged in a hexagonal structure. In these solids atomselements form the molecule which is further joined by a nonpolar bond to form this kind of molecular solid.
Amorphous solids are made of rubber glass and sulphur. A Polmers B Metals c Ceramics D All of the above E None of the above. A crystal or crystalline solid is a solid material whose constituents such as atoms molecules or ions are arranged in a highly ordered microscopic structure forming a crystal lattice that extends in all directions.
B None of these. These include all metals many ceramics and some polymers which will be semicrystalline. This is why they do not have edges like crystals do.
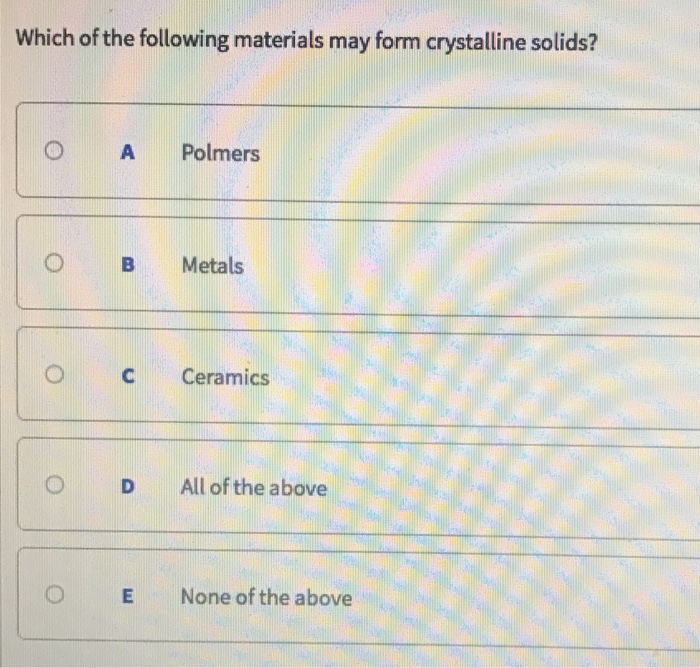
A Metals b Polymers c Ceramics d Both a and b e Both b and c f Both a and c g All of a b and c h None of the above. A All of these. Which of the following materials may form crystaline solids.
When an element exist in more than one crystalline form this is called allotropy and crystalline forms are called allotropes or allotropic forms. Organic substances like benzoic acid oxalic acid camphor naphthalene etc are also crystalline solids. Polymers ceramics and metals Which crystal systems listed below has have the following relationship for the unit.
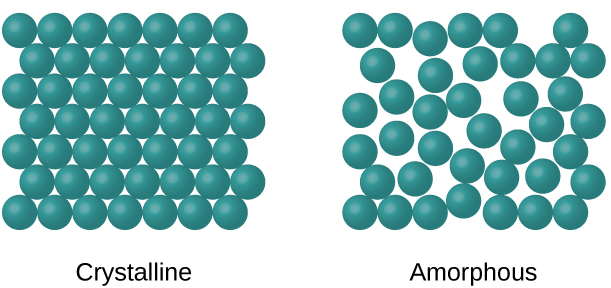
The entities of a solid phase may be arranged in a regular repeating pattern crystalline solids or randomly amorphous. Which of the following materials may form crystalline solids.
12 4 The Fundamental Types Of Crystalline Solids Chemistry Libretexts
Solved 15 Which Of The Following Materials May Form Chegg Com

11 7 Structure Of Solids Chemistry Libretexts Unit Cell Material Science Physical Chemistry
Solved Which Of The Following Materials May Form Crystalline Chegg Com
Crystalline Solids Amorphous Solids Definition Examples
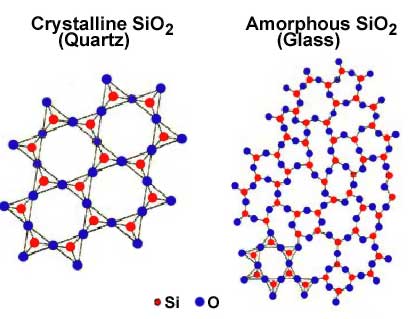
Nondestructive Evaluation Physics Materials

Spirit Stone Quiz Which Stone Is Connected To Your Spirit How Do Crystals Form Crystals Stone
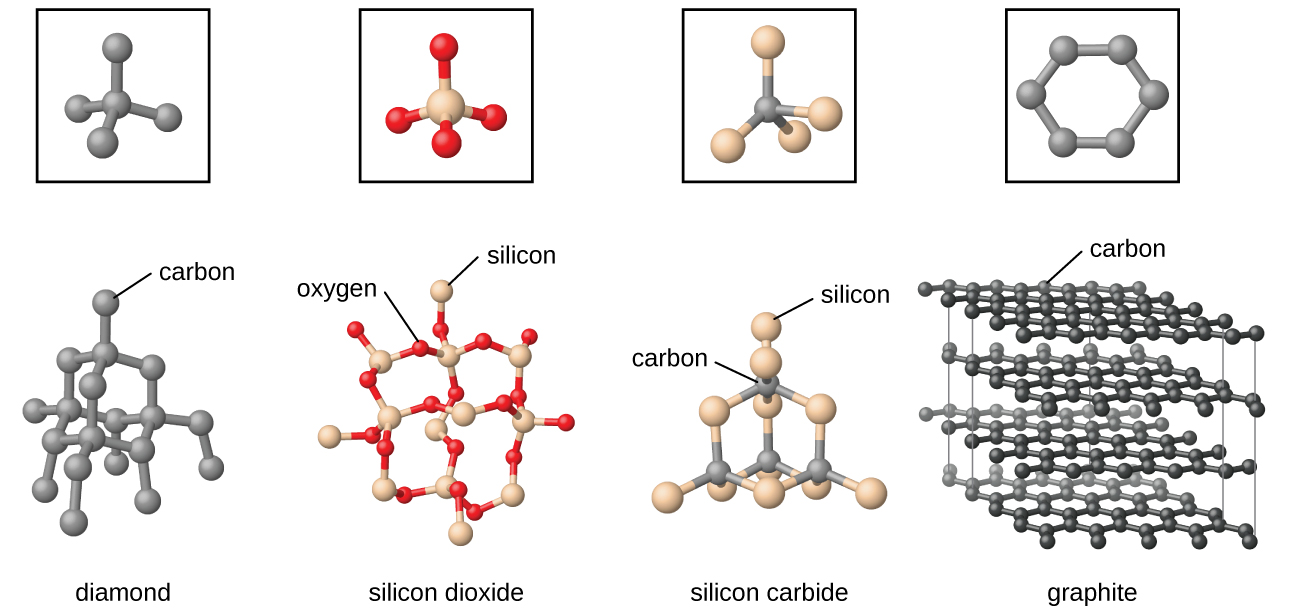
10 5 The Solid State Of Matter Chemistry

Simon Horn S Handsome Obelisk Four Poster Bed Simonhorn Com Telegraph Magazine October 31st 2015 House Design Design Home

Cactus Quartz Spirit Quartz Fairy Quartz Light Amethyst Etsy Spirit Quartz Amethyst Purple Chalcedony
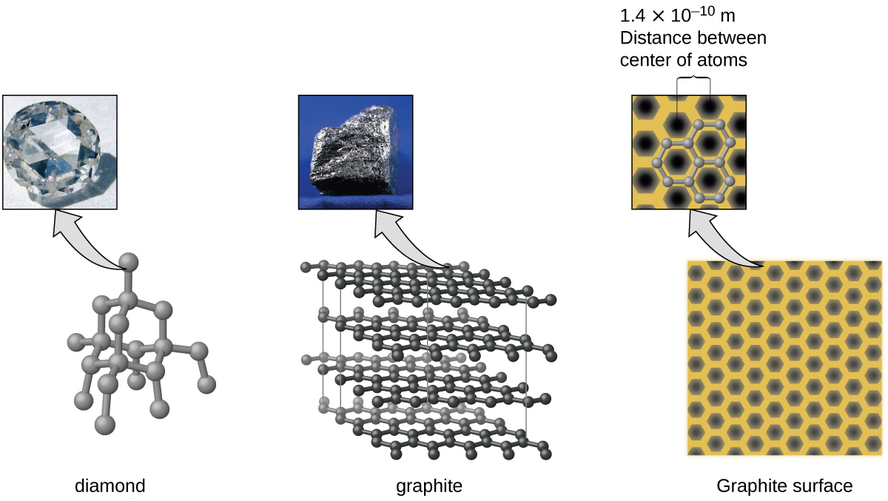
12 4 The Fundamental Types Of Crystalline Solids Chemistry Libretexts
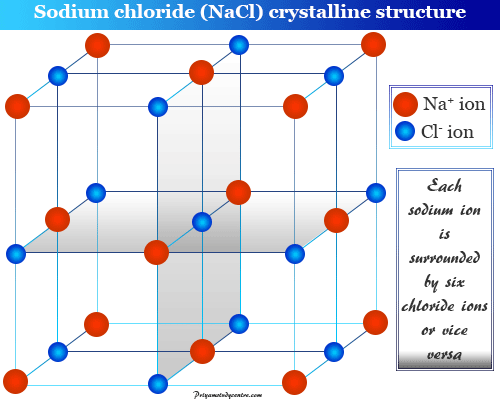
Crystalline Solids Amorphous Solids Definition Examples

The Solid State Of Matter Chemistry Atoms First

12 Bis Triethoxysilyl Ethane Is A Non Functional Silane And A Double Trialkoxysilyl Precursor It Is Used As A Plating Agent 1 Chemistry Molecules Being Used
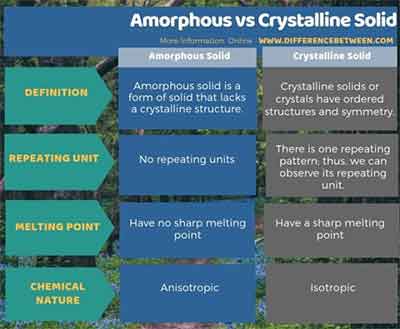
Differences Between Amorphous Crystalline Solids

The Solid State Of Matter Chemistry Atoms First

Types Of Crystalline Solids And Their Properties By Chemistry Topics Medium

Comments
Post a Comment